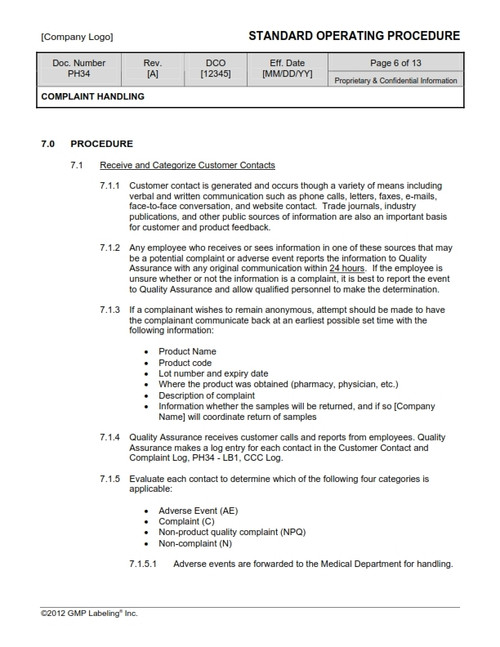
MD34 COMPLAINT HANDLING SOP Template
MSRP:
Was:
Now:
$189.00
(You save
)
Medical Device Standard Operating Procedure Template- Describes the process for receiving, reviewing and evaluating complaints and describes the responsibilities associated with the complaint handling process. This procedure also describes elements of the customer communication and feedback process. Package consists of the procedure, a Complaint Report form and a Customer Contact and Complaint Log.
Questions?
M - F | 9 AM - 5 PM EST

Need Custom Quality Control Labels?
Thousands of Color, Shape and Material Combinations Available and designed for your specific application.
Get StartedWhy Choose GMP Labeling?
The premier provider of compliance identification products to Pharmaceutical and Medical Device manufacturers, or any other business that involves precision and quality control.
ISO 9001 Certified
Certified under ISO 9001 and experts in GMP standards for over 30 years.
Solution Partner
A personalized approach as your labeling solution partner.
Domestic Manufacturing
Proud to be a Women's Owned Business with domestic manufacturing.
Related Products


Add to Cart
The item has been added

Add to Cart
The item has been added

Add to Cart
The item has been added

Add to Cart
The item has been added

Add to Cart
The item has been added

Add to Cart
The item has been added

Add to Cart
The item has been added
!




 Need Help?
Need Help?